Ever wondered how sunlight powers your house or how a motion-sensor light flicks on when you walk by? While these marvels might seem like magic, they’re actually the result of two key scientific phenomena: the photovoltaic effect and the photoelectric effect. Despite sounding similar—and both involving light and electrons—they’re as different as night and day in how they work and what they accomplish.
What Is the Difference Between the Photovoltaic Effect and the Photoelectric Effect?
Let’s start with a quick definition of both terms before we dig into the details:
- Photovoltaic Effect: This is the process by which sunlight is converted directly into electricity using special materials called semiconductors. Think solar panels.
- Photoelectric Effect: This is when light hits a material, usually metal, and knocks out electrons from its surface. Think motion detectors or how Einstein revolutionized physics.
To make it even clearer, here’s a quick comparison table that sums up their key differences:
| Feature | Photovoltaic Effect | Photoelectric Effect |
|---|---|---|
| Main Process | Converts sunlight into electricity | Knocks electrons out of a material’s surface |
| Energy Source | Sunlight (or other light sources) | High-frequency light (e.g., UV or blue light) |
| Materials Involved | Semiconductors (e.g., silicon) | Metals (e.g., zinc, copper) |
| Key Application | Solar cells and renewable energy | Sensors, photocells, and quantum mechanics research |
| Threshold Frequency | No strict threshold frequency | Requires light above a specific frequency |
What Is the Photovoltaic Effect?
The photovoltaic effect is the fundamental process that makes solar panels work. It’s what allows us to take sunlight—a completely free and abundant energy source—and transform it into electricity. This effect occurs when light shines on a semiconductor material, such as silicon, creating an electric current.
Historically, the photovoltaic effect was discovered by French physicist Edmond Becquerel way back in 1839. While experimenting with an electrolytic cell made of two metal electrodes and a conductive solution, he noticed that light increased the electricity flowing through the cell. Fast forward to the modern era, and we’ve turned this simple observation into cutting-edge technology that powers everything from homes to satellites.
What’s especially cool about the photovoltaic effect is that it doesn’t rely on any moving parts, chemical reactions, or combustion, making it one of the cleanest and most sustainable methods for generating power. It’s literally electricity, on demand, from the sun.
How Does the Photovoltaic Effect Work?
The photovoltaic effect boils down to three main steps, all of which occur inside a solar cell:
- Absorption of Light Energy
When sunlight hits a solar panel, the light is absorbed by the semiconductor material inside, typically silicon. This light comes in packets of energy called photons. The energy of these photons excites the electrons in the silicon, essentially waking them up and giving them enough energy to move freely. - Creation of Electron-Hole Pairs
In semiconductors, an electron that gains energy leaves behind a “hole” (a space where an electron used to be). This process creates what’s called an electron-hole pair. The free electrons carry a negative charge, while the holes carry a positive charge, creating the potential for an electric current. - Separation of Charges and Current Flow
Inside the solar cell, there’s an electric field that acts like a traffic cop. It pushes the electrons in one direction and the holes in the opposite direction. This movement of charges creates a flow of electricity, which can then be captured and used to power your home or charge your phone.
Here’s a simplified diagram of the process:
[Sunlight] → [Photon Absorption] → [Excited Electrons] → [Electric Current]
It’s worth noting that the efficiency of this process depends on several factors, such as the type of semiconductor material used, the quality of the solar panel, and even the angle and intensity of sunlight.
Where Is the Photovoltaic Effect Used?
The photovoltaic effect is the backbone of solar energy technology, one of the fastest-growing renewable energy sources in the world. Solar panels, which are essentially arrays of photovoltaic cells, can be found on rooftops, in large solar farms, and even in outer space powering satellites.
Beyond generating electricity for homes and businesses, photovoltaic technology is also used in:
- Portable Solar Chargers: Devices that let you charge gadgets using sunlight.
- Solar-Powered Vehicles: Think solar-powered cars, boats, and even planes.
- Solar Streetlights: Lights that store energy during the day and illuminate streets at night.
Fun fact: Solar power accounted for over 1,000 GW of installed capacity worldwide by 2023, making it a key player in the global push for clean energy.

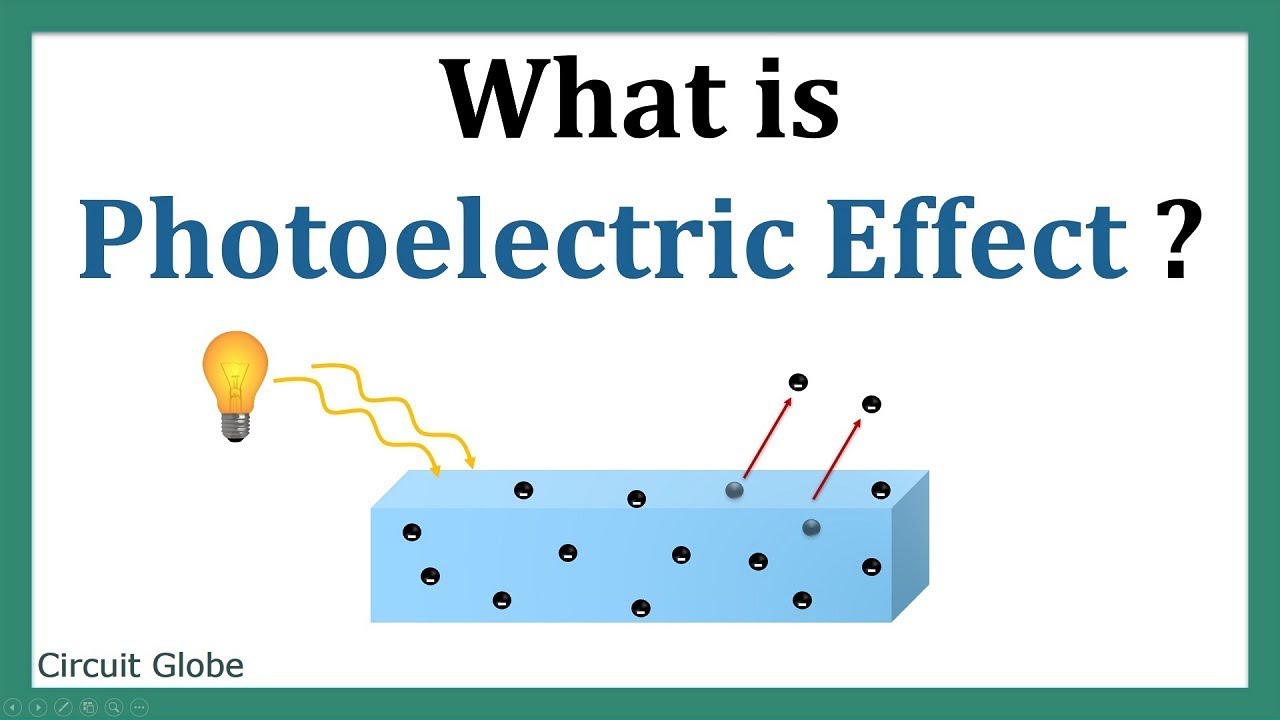
What Is the Photoelectric Effect?
If the photovoltaic effect is the backbone of solar panels, the photoelectric effect is the cornerstone of modern physics. Discovered in 1887 by German physicist Heinrich Hertz and later explained by Albert Einstein in 1905, the photoelectric effect occurs when light hits a material (typically a metal) and knocks electrons out of its surface.
The key here is that the light doesn’t just heat up the material or create a general disturbance—it actually ejects individual electrons, which can then be collected to create a flow of electric current. This process gave humanity its first concrete evidence that light behaves not just as a wave, but also as a particle (hello, photons!).
Einstein’s work on the photoelectric effect was so revolutionary that it earned him the Nobel Prize in Physics in 1921. So if you’ve ever wondered why Einstein is so celebrated, this discovery was one of the reasons.
How Does the Photoelectric Effect Work?
To understand the photoelectric effect, let’s break it down step by step:
- Light Strikes a Material
When light (composed of photons) hits the surface of a material, the energy of the photons is transferred to the electrons in the material. - Photon Energy is Absorbed
The electrons absorb this energy, but here’s the catch: not just any photon will do. The energy of the photon must be greater than the material’s work function—the minimum amount of energy required to knock an electron free. For instance, ultraviolet light (which has high energy) is more likely to cause the photoelectric effect than red light (which has lower energy). - Ejection of Electrons
If the photon’s energy is sufficient, the electron absorbs the energy, overcomes the work function, and is ejected from the surface of the material. These freed electrons are called photoelectrons. - Collection of Electrons
The photoelectrons can then be collected, and their flow creates an electric current, which can be measured or used in devices.
Here’s a helpful analogy: Imagine a pinball machine. The photon is like the plunger launching the ball (the electron) out of the machine. If the plunger isn’t strong enough (low energy), the ball stays put. But if it has enough power, the ball is launched into motion—just like electrons in the photoelectric effect.
Applications of the Photoelectric Effect
The photoelectric effect has far-reaching applications in both technology and science. Here are some real-world uses that you might not have realized rely on this phenomenon:
- Photocells (Light Sensors): Used in automatic doors, streetlights, and motion detectors. These sensors detect changes in light and use the photoelectric effect to trigger a response.
- Solar Panels (Partially): While solar panels mainly rely on the photovoltaic effect, the photoelectric effect plays a minor role in specific thin-film technologies.
- Television and Cameras: Early cathode ray tubes and modern cameras use principles derived from the photoelectric effect.
- Quantum Research: The photoelectric effect was instrumental in developing quantum mechanics and understanding the dual nature of light.
- X-Ray and UV Detectors: Devices that measure high-energy light (like X-rays) depend on the photoelectric effect to detect and measure radiation.
Fun Fact: Einstein and the Photoelectric Effect
You might assume Einstein’s fame comes from E=mc² (and you’re not wrong), but his work on the photoelectric effect arguably had a bigger impact on our day-to-day lives. It’s why your automatic doors open at the grocery store and why your camera can capture those perfect sunset photos.
Differences Between the Photovoltaic Effect and the Photoelectric Effect
While the photovoltaic effect and the photoelectric effect both involve light interacting with materials to generate electricity, they operate on fundamentally different principles. Let’s break it down step by step to understand their unique characteristics and where they diverge.
Mechanisms of Action
- Photovoltaic Effect: In the photovoltaic effect, light energizes electrons within a semiconductor material, creating electron-hole pairs. These pairs are separated by an internal electric field, resulting in a flow of current. The electrons don’t leave the material but instead move through it, creating usable electricity.
- Photoelectric Effect: In the photoelectric effect, photons (light particles) physically knock electrons out of a material’s surface. These ejected electrons, called photoelectrons, can be captured to generate current. This is a surface-level interaction, unlike the bulk processes in the photovoltaic effect.
Think of it this way: The photovoltaic effect is like a controlled traffic system inside the material, while the photoelectric effect is more like a game of pool, where photons hit electrons and knock them out entirely.
Energy Requirements and Thresholds
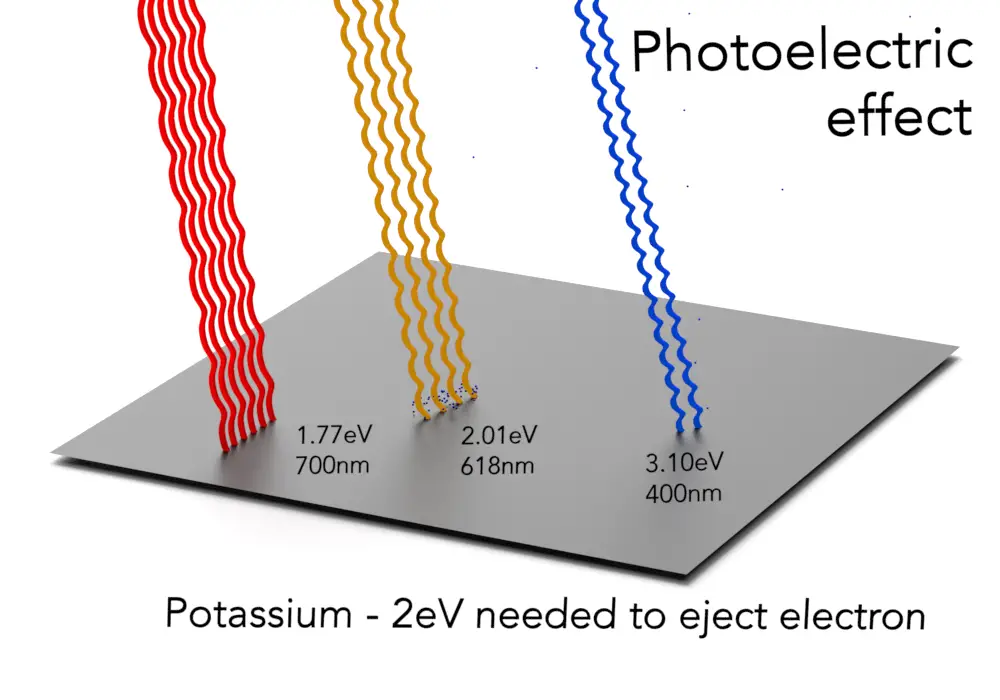
- Photovoltaic Effect: There is no strict minimum energy threshold for photons. As long as the material can absorb the light and generate electron-hole pairs, electricity can be produced. This makes the photovoltaic effect more flexible in terms of the spectrum of light it can use, including visible and infrared light.
- Photoelectric Effect: The photoelectric effect requires photons with energy greater than the material’s work function. For example, ultraviolet light, which has higher energy, is often required to eject electrons from metals like zinc. If the photon’s energy is too low (like red light), nothing happens—no matter how bright the light is.
Light Energy Utilization
- Photovoltaic Effect: It converts the energy from light into electrical energy efficiently. The focus is on generating and sustaining a current that can power devices. This makes it the driving force behind renewable energy solutions like solar panels.
- Photoelectric Effect: The light energy is used to eject electrons. While it can create a measurable current, its purpose is typically for detection or measurement, such as in light sensors or scientific instruments.
Applications and Use Cases
Here’s where these effects really show their distinct roles in technology:
| Application | Photovoltaic Effect | Photoelectric Effect |
|---|---|---|
| Solar Energy | Primary mechanism for solar panels and photovoltaic cells. | Not a primary application for generating solar energy. |
| Sensors | Not used for sensing. | Key to photocells, light sensors, and motion detectors. |
| Quantum Mechanics | Indirectly contributes to understanding light-matter interactions. | Fundamental to the discovery of quantum physics. |
| Electronics | Used in powering devices sustainably. | Used in detecting or amplifying light signals. |
For example, the photovoltaic effect is why your rooftop solar panels can power your coffee maker, while the photoelectric effect is why your motion-sensor lights switch on when you walk into a room at night.
Role in Scientific Discovery
The photoelectric effect played a pivotal role in the development of quantum mechanics, proving that light isn’t just a wave but also behaves as a particle. This discovery changed the way we understand the universe. On the other hand, the photovoltaic effect has driven innovation in renewable energy, helping to combat climate change and reduce dependence on fossil fuels.
Photovoltaic vs. Photoelectric
To summarize:
- The photovoltaic effect is all about creating an electric current inside a material when light is absorbed, making it vital for solar energy.
- The photoelectric effect is about freeing electrons from a material’s surface when light strikes it, a key principle for quantum mechanics and light-based sensors.
- Both phenomena showcase the incredible power of light and how humanity has harnessed it for technology and discovery.